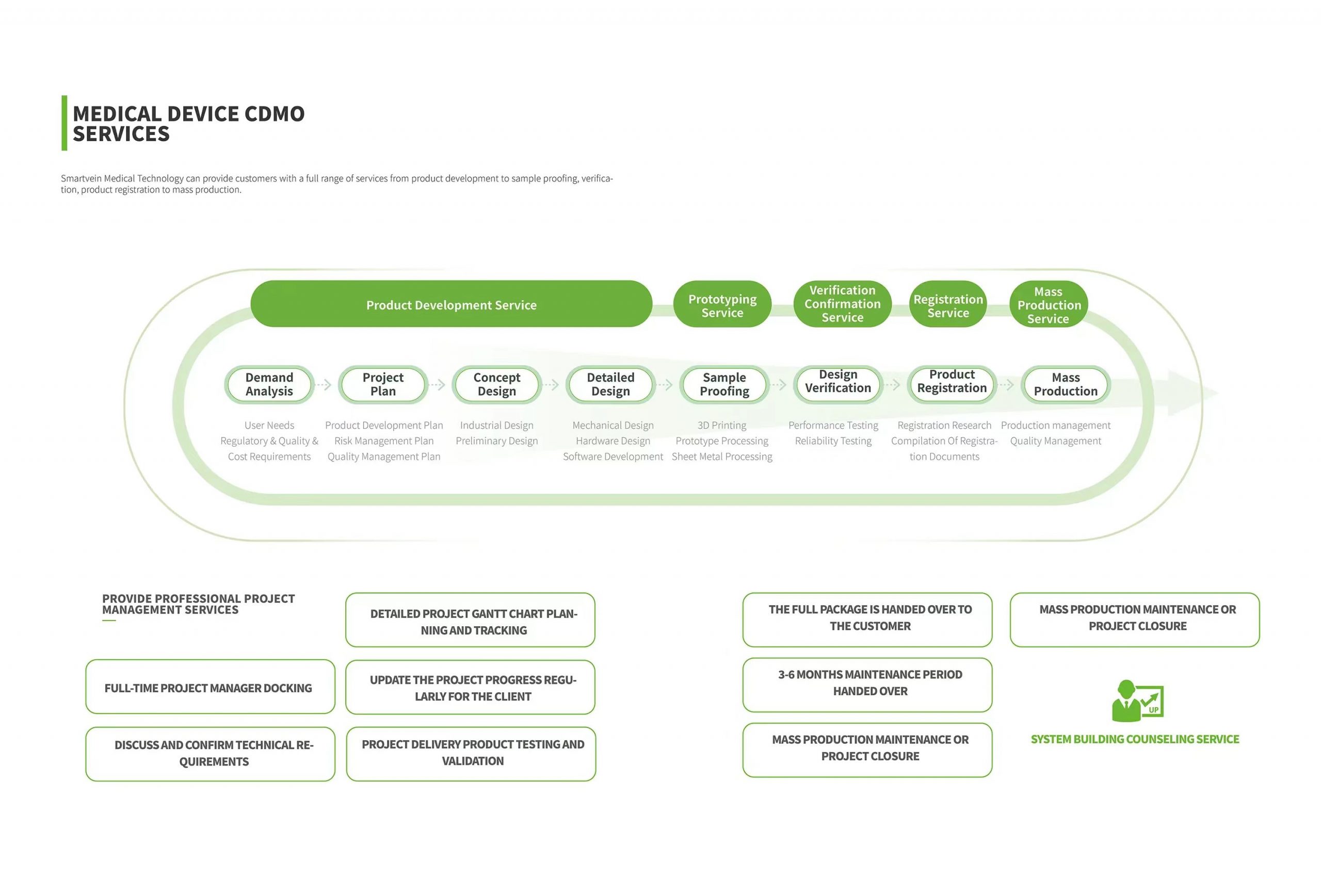
Description

Product development service
Sample proofing service
Validation service
Registration service
Mass production service
01 Requirement Analysis
User needs, regulatory needs
Quality requirements, cost requirements
02 Project plan
Product development plan
Risk management plan
Quality control plan
03 Conceptual design
lndustrial design
Preliminary design scheme
04 Detailed Design
Structural design
Hardware design
Software design
05 Sample making
3D printing
Hand plate processing
Sheet metal working
06 Design verification
Performance test
Security testing
07 Product registration
Registration research
Registration strategy planning
Registration document writing
08 Mass production
Production management
Quality control
PROVIDE PROFESSIONAL PROJECTMANAGEMENT SERVICES
Detailed project Gantt chart planning and tracking
Full-time project manager docking
Update the project progress regularly for the client
Discuss and confirm technical requirements
Project delivery product testing and validation
System building counseling service
The full package is handed overto the customer
Mass production maintenance or project closure
3-6 months maintenance period handed over
Mass production maintenance or project closure




Reviews
There are no reviews yet.